
Le focene comuni
Sebbene le focene comuni siano la specie di cetaceo più abbondante nelle acque del Regno Unito 248 e si ritenga che le popolazioni siano stabili 249, questa specie è altamente minacciata altrove 250 ed è molto vulnerabile al disturbo, all'inquinamento e alla perdita di fonti di cibo. Le attività legate al petrolio e al gas contribuiscono in modo determinante agli impatti cumulativi su queste piccole energiche creature, portandole potenzialmente alla fame poiché sprecano l’energia per rispondere ai disturbi. Le focene comuni sono sottoposte ad un'ulteriore pressione da parte degli inquinanti 251, molti dei quali provenienti dall'industria petrolifera e del gas 252, che si bioaccumulano e possono anche essere trasmessi ai piccoli nel latte materno 253. I rilievi sismici in Scozia hanno portato a cambiamenti significativi nel comportamento delle focene comuni fino a 25 km dal sito di rilevamento 89 e il rumore dei cantieri ha portato le focene a spostarsi fino a 20 km fuori da un'area per evitare il disturbo 254. Si è dimostrato che il rumore dei rilievi e dei cantieri riduce la capacità delle focene di localizzare efficacemente le prede. Un recente studio sull'impatto della costruzione di una nuova piattaforma di gas sulla Dogger Bank ha mostrato importanti cambiamenti nel numero e nell'attività delle focene che sono continuati per mesi 255.
sottoposte ad un'ulteriore pressione da parte degli inquinanti 251, molti dei quali provenienti dall'industria petrolifera e del gas 252, che si bioaccumulano e possono anche essere trasmessi ai piccoli nel latte materno 253. I rilievi sismici in Scozia hanno portato a cambiamenti significativi nel comportamento delle focene comuni fino a 25 km dal sito di rilevamento 89 e il rumore dei cantieri ha portato le focene a spostarsi fino a 20 km fuori da un'area per evitare il disturbo 254. Si è dimostrato che il rumore dei rilievi e dei cantieri riduce la capacità delle focene di localizzare efficacemente le prede. Un recente studio sull'impatto della costruzione di una nuova piattaforma di gas sulla Dogger Bank ha mostrato importanti cambiamenti nel numero e nell'attività delle focene che sono continuati per mesi 255.
Anche le chiazze di petrolio in superficie rappresentano un serio problema per le focene poiché devono tornare in superficie ogni 5 minuti circa per respirare. I loro sfiatatoi possono facilmente contaminarsi con petrolio, vapori tossici e altri inquinanti provenienti dalla superficie 231. Il loro comportamento può anche esacerbare gli impatti dell’inquinamento perché potrebbero non spostarsi dalla loro area di alimentazione, continuando a nutrirsi in aree altamente inquinate e ad ingerire prede inquinate 256. Possono inoltre subire ulteriori disturbi a causa delle attività di bonifica delle fuoriuscite di petrolio e degli effetti tossici delle sostanze utilizzate per disperdere il petrolio 257.
Anche in assenza di una fuoriuscita di grandi dimensioni, le focene subiscono gli effetti negativi delle sostanze chimiche utilizzate nel processo di perforazione, e che vengono rilasciate dalle attività storiche quando i siti vengono dismessi o altrimenti disturbati. Gli studi hanno scoperto che è molto probabile che i PCB (ora vietati ma in precedenza ampiamente utilizzati nel settore del petrolio e del gas offshore, e ancora rilasciati) vengano trasmessi ai cuccioli di focena mentre si nutrono dalle loro madri 253. La combinazione di contaminanti trasmessi ai cuccioli è particolarmente potente come insieme di neurotossine e probabilmente ha un impatto sullo sviluppo dei giovani 258. Poiché le focene comuni sono numerose e svolgono un importante ruolo ecologico, 259 questi molteplici impatti non colpiscono solo questa affascinanti creature, ma creano un effetto a cascata sull'intero ecosistema.
2.2 Perdita di habitat
Quando vengono costruite piattaforme petrolifere, piattaforme per il gas e relativi oleodotti, gasdotti, cavidotti e altre infrastrutture, si verifica una perdita diretta di habitat 28. Molte installazioni offshore, come le unità mobili di perforazione, possono anche essere ancorate al fondale marino, con conseguente perdita di habitat e rischio di ulteriori danni, ad esempio se le ancore iniziano a trascinarsi durante forti tempeste 70.
Poiché l'area persa a causa di queste strutture appare relativamente piccola, è stata spesso ignorata dagli imprenditori e dalle autorità di regolamentazione. Tuttavia, la perdita di habitat si verifica comunque e non è limitata all'area immediatamente circostante l'insediamento, ma può estendersi per almeno 500 metri dall'installazione 71. Ciò accade perché la costruzione e le trivellazioni creano sedimentazione,72 spostando fango e sabbia, che possono formare spessi strati sul fondale marino circostante. Questa sedimentazione può soffocare le creature marine che costruiscono l'habitat e portare alla completa perdita o a un grave degrado degli habitat 22. La perdita di habitat si verifica anche a causa dell'inquinamento. L'inquinamento da petrolio, ad esempio, può portare a una perdita di habitat su larga scala e a lungo termine, mentre i detriti di trivellazione e altri inquinanti chimici possono avere un impatto sugli organismi che costruiscono l'habitat, come i mitili, degradando la diversità degli habitat che creano.
 La perdita di habitat dovuta alle infrastrutture petrolifere e del gas è sempre significativa, ma è motivo di particolare preoccupazione quando ha un impatto sulle Aree Marine Protette (AMP), sugli habitat o sulle specie rare o sugli ecosistemi marini vulnerabili. Un esempio calzante sono le comunità di spugne profonde dell’AMP Faroe-Shetland Sponge Belt. Questo habitat è attualmente valutato ‘in condizioni sfavorevoli’ 73 –termine tecnico per indicare il mancato raggiungimento degli obiettivi di conservazione– e dovrebbero essere in corso degli sforzi per recuperarlo. Tuttavia, l'esplorazione e l'estrazione di idrocarburi sono già in corso e altri siti vengono attualmente resi disponibili in aree di questo habitat o nelle vicinanze, tra cui gli enormi giacimenti petroliferi di Rosebank e Cambo.
La perdita di habitat dovuta alle infrastrutture petrolifere e del gas è sempre significativa, ma è motivo di particolare preoccupazione quando ha un impatto sulle Aree Marine Protette (AMP), sugli habitat o sulle specie rare o sugli ecosistemi marini vulnerabili. Un esempio calzante sono le comunità di spugne profonde dell’AMP Faroe-Shetland Sponge Belt. Questo habitat è attualmente valutato ‘in condizioni sfavorevoli’ 73 –termine tecnico per indicare il mancato raggiungimento degli obiettivi di conservazione– e dovrebbero essere in corso degli sforzi per recuperarlo. Tuttavia, l'esplorazione e l'estrazione di idrocarburi sono già in corso e altri siti vengono attualmente resi disponibili in aree di questo habitat o nelle vicinanze, tra cui gli enormi giacimenti petroliferi di Rosebank e Cambo.
Entrambi i giacimenti richiederebbero oleodotti e altre infrastrutture nell'AMP, mettendo a rischio le comunità di spugne. Esistono già chiare prove della completa perdita di alcuni habitat di spugne e della mancanza di recupero dopo le trivellazioni nel giacimento di Laggan, che si trova al centro dell'area protetta 74. I giacimenti petroliferi di Rosebank e Cambo richiederebbero la costruzione di oleodotti e altre infrastrutture nell'Area Marina Protetta, mettendo a rischio le comunità delle spugne.
2.3 Rumore
Le industrie petrolifere e del gas offshore sono una delle principali fonti di rumore oceanico 75, i cui impatti sono spesso minimizzati o sottovalutati, ma hanno gravi ripercussioni sugli ecosistemi del Regno Unito. Una crescente cacofonia di rumore marino (soprannominata "antropofonia") 76, dal rombo dei motori delle barche alle esplosioni sottomarine, ha un impatto enorme sulla vita marina 77. Analogamente ad altri tipi di inquinamento acustico marino, ha un impatto transfrontaliero e cumulativo 78. L'entità e la durata dell'impatto del rumore associato al petrolio e al gas sono difficili da misurare, ma influenzano interi ecosistemi e le future generazioni di animali marini 79. Particolarmente preoccupanti sono le indagini sismiche con airgun condotte per individuare risorse di petrolio e gas, che sono tra i suoni antropogenici più forti 82. Queste sono molto più intrusive di quelle utilizzate per altri altri progetti, come i parchi eolici 80. Comportano intensi impulsi sonori 81 che possono essere rilevati a 4000 km di distanza 83. Il rumore che altera il comportamento [della fauna marina, ndt] si estende per migliaia di chilometri quadrati attorno a ogni indagine sismica 84.
Per i mammiferi marini ciò può comportare impatti fisici diretti, tra cui la perdita dell'udito nei delfini tursiopi 85, che ha gravi implicazioni per animali altamente dipendenti dal suono 86, o in casi estremi, la morte 87. Ciò può indurli a ridurre l'ecolocalizzazione che utilizzano per comunicare 16, a lasciare buone aree di alimentazione 88, a ridurre l'attività di caccia 89 e a sprecare energie preziose nello spostamento su lunghe distanze per evitare il rumore, distogliendo tale energia dalla riproduzione e quindi avendo un impatto anche sulle generazioni future 90. L’analisi su larga scala delle indagini sismiche nel Regno Unito ha mostrato una diminuzione degli avvistamenti di mammiferi marini a seguito dell'attività sismica, con le focene e i capodogli che mostrano una maggiore sensibilità 91,92. Anche le balenottere minori reagiscono allontanandosi 92 e le megattere cambiano il loro comportamento ed evitano l'attività sismica, con una distanza fino a 12 km 94. Il suono sismico può modificare importanti comportamenti migratori tanto da fare in modo che i mammiferi marini si trovino nel posto sbagliato al momento sbagliato e perdano opportunità di nutrirsi o riprodursi. I delfini tursiopi, ad esempio, sono passati dalla loro normale dieta a base di pesce a nutrirsi di spugne dei fondali marini 85 per evitare il rumore sismico. Quanto più piccolo è un mammifero marino, tanto più delicato è l’equilibrio tra l’energia derivata dal cibo e l’energia necessaria per sopravvivere. Ad esempio, aggiungere deviazioni inutili alla vita quotidiana di una focena comune può avere conseguenze significative, tra cui l'aumento del rischio di fame 95.
Mentre le ricerche più datate hanno permesso di comprendere l'importanza del rumore per balene e delfini, l'impatto del rumore antropogenico su altre forme di vita marina è stato appena compreso e indagato 75. Ad esempio, stanno emergendo ricerche sull'importanza dell'udito nelle tartarughe e sul potenziale impatto delle indagini sismiche e di altri rumori marini 96. Anche gli invertebrati possono essere colpiti, ad esempio le larve di capesante hanno mostrato gravi deformità fatali a seguito di impatti sismici 97, e nei calamari giganti sono stati riscontrati danni ai tessuti, agli organi e ai loro importanti statoliti sensoriali 98. Effetti negativi legati al suono sismico sono stati rivelati anche da studi su granchi, seppie 99, aragoste 100, cozze, polpi 101, calamari 98 e molte altre specie, e un anno dopo l'impatto sismico è stato registrato un recupero limitato dagli effetti 100.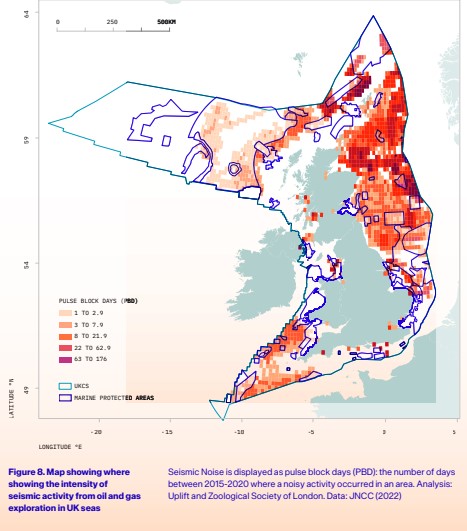
Una nuova analisi di Uplift (Figura 8) evidenzia come la frequenza e l'intensità dell'inquinamento acustico associato alle indagini sismiche su petrolio e gas siano elevate nelle acque del Regno Unito, e come persino alcune delle aree marine protette siano soggette a rumore eccessivo per lunghi periodi. Una volta identificate le riserve offshore di petrolio e gas, un ulteriore inquinamento acustico è associato alla costruzione e alla perforazione di pozzi esplorativi, nonché all'installazione e alla rimozione delle infrastrutture al termine del loro ciclo di vita 28. I principali impatti associati alla costruzione sono il rumore di perforazione e di palificazione delle fondamenta, e i disturbi associati all'aumento del traffico marittimo e alla movimentazione delle attrezzature. Questo tipo di rumore provoca alterazioni nel comportamento comunicativo nei delfini e in altre specie di mammiferi marini.
Il rumore prodotto dai cantieri può causare lo spostamento delle focene comuni di circa 20 km 109,110. Il rumore sottomarino è generato dalle piattaforme di produzione e dalle attività operative, tra cui perforazione, traffico navale e posa di condotte. Laddove la piattaforma di perforazione o di produzione dipenda dal supporto e dall'approvvigionamento di altre navi di riserva e di rifornimento, queste sono spesso dotate di propulsori a posizionamento dinamico e motori potenti e pertanto contribuiscono al livello di rumore complessivo delle attività di perforazione e produzione 111. Lo sviluppo e l'attività di estrazione di petrolio e gas comportano inevitabilmente un aumento complessivo del rumore marino nelle immediate vicinanze, ma anche in aree molto più ampie.
Le vongole oceaniche
Le vongole oceaniche sono straordinarie sentinelle del cambiamento climatico dalla lunga vita e sono una specie prioritaria per la conservazione 278 e la designazione di aree marine protette 279–282, ma sono minacciate dagli attuali 283 e dai nuovi sviluppi petroliferi e del gas offshore 284, che portano alla perdita di habitat e all'accumulo di contaminazione 285. Questi molluschi bivalvi, dal guscio spesso, si trovano in fondali sabbiosi e ghiaiosi fino a circa 500 metri 286 e sono noti soprattutto per la loro notevole longevità 287. Un esemplare islandese aveva 507 anni, il che lo rende l'animale ‘non coloniale’ [nel senso che non vive in comunità di organismi, ndt] più longevo. Individui secolari sono stati regolarmente registrati nelle acque del Regno Unito 288. La loro notevole età e il fatto che depongono anelli di crescita annuali - che forniscono informazioni sull'ambiente in cui hanno vissuto - li rende estremamente utili nello studio della storia ambientale 289,290 e nella scienza dei cambiamenti climatici 291,292. Sono anche utilizzati come indicatori della salute ambientale, ad esempio fornendo informazioni sull'accumulo di tossine (come i metalli pesanti) attraverso le concentrazioni nei loro gusci e nella loro carne 285, o per la loro preferenza per i sedimenti meno contaminati 293.
 La loro lenta crescita, il tempo impiegato per raggiungere la maturità e la longevità contribuiscono a rendere questa specie vulnerabile agli impatti antropici 286 e potrebbero volerci decenni o addirittura secoli prima che le popolazioni si riprendano. Sono una specie minacciata o in declino secondo l'OSPAR 294, sono una "caratteristica di interesse conservazionistico" per la designazione di Zona di Conservazione Marina nelle acque del Regno Unito 279, e sono in grave pericolo di estinzione nel Mar Baltico 250. La vongola oceanica può presentarsi in densità molto basse, ma forma anche banchi densi dove possono trovarsene a centinaia in un metro quadrato, e il Mare del Nord presenta alcune delle densità più elevate registrate al mondo 295.
La loro lenta crescita, il tempo impiegato per raggiungere la maturità e la longevità contribuiscono a rendere questa specie vulnerabile agli impatti antropici 286 e potrebbero volerci decenni o addirittura secoli prima che le popolazioni si riprendano. Sono una specie minacciata o in declino secondo l'OSPAR 294, sono una "caratteristica di interesse conservazionistico" per la designazione di Zona di Conservazione Marina nelle acque del Regno Unito 279, e sono in grave pericolo di estinzione nel Mar Baltico 250. La vongola oceanica può presentarsi in densità molto basse, ma forma anche banchi densi dove possono trovarsene a centinaia in un metro quadrato, e il Mare del Nord presenta alcune delle densità più elevate registrate al mondo 295.
La vongola oceanica è una specie settentrionale che si trova all'estremità meridionale del suo areale, nel Mare del Nord centrale, e non si estende fino a sud, ed è quindi sensibile agli aumenti di temperatura associati ai cambiamenti climatici 296. Essa svolge un ruolo importante nella produttività degli ecosistemi sabbiosi e ghiaiosi ed è anche un'importante fonte di cibo per il merluzzo bianco 297 e altre specie. È vulnerabile all'inquinamento chimico associato all'industria petrolifera e del gas offshore e, data la sua lunga durata di vita, è particolarmente suscettibile a inquinanti persistenti e accumulabili come IPA e metalli pesanti 285. L'impatto acustico della vongola oceanica non è stato studiato, ma l'evidenza dell'impatto del suono delle prospezioni sismiche su altri bivalvi 97 evidenzia il potenziale impatto significativo sullo sviluppo. I rischi per le aggregazioni di vongole oceaniche sono stati evidenziati in diverse Aree Marine Protette con presenza di attività petrolifera e del gas offshore, tra cui l'AMP della Cintura delle Spugne delle Isole Faroe 298 e l'AMP del Canale delle Isole Faroe-Shetland nord-orientali, dove le vongole sono state osservate entro 50 metri dall'infrastruttura idrocarburica 263.
(3. Continua)
* Traduzione di Ecor.Network
In Deep Water: Exposing the hidden impacts of oil and gas on the UK’s seas
Oceana, Uplift
Aprile 2023, 45 pp.
Download:

Note:
1. Galparsoro, I. et al. Reviewing the ecological impacts of offshore wind farms. npj Ocean Sustainability 1, 1 (2022).
2. Marappan, S., Stokke, R., Malinovsky, M.P., & Taylor, A. Assessment of impacts of the offshore oil and gas industry on the marine environment. in OSPAR, 2023: The 2023 Quality Status Report for the North-East Atlantic. (OSPAR Commission, 2022).
3. Welsby, D., Price, J., Pye, S. & Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 597, 230–234 (2021).
4. IEA. Net Zero by 2050. https://www.iea.org/reports/net-zero-by-2050 (2021).
5. BEIS/Prime Minister’s Office. UK enshrines new target in law to slash emissions by 78% by 2035. https://www.gov.uk/government/news/uk-enshrines-new-target-in-law-to-slash-emissions-by-78-by-2035 (2021).
6. UNFCCC. The Paris Agreement. https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (Undated).
7. Supran, G., Rahmstorf, S. & Oreskes, N. Assessing ExxonMobil’s global warming projections. Science 379, eabk0063.
8. Franta, B. Early oil industry disinformation on global warming. Environmental Politics 30, 663–668 (2021).
9. ExxonMobil. https://www.exxonmobil.co.uk/company/overview/uk-operations/production. (2019).
10. McCauley, D. J. et al. Marine defaunation: Animal loss in the global ocean. Science 347, 1255641 (2015).
11. Worm, B. et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 314, 787–790 (2006).
12. Palumbi, S. R. et al. Managing for ocean biodiversity to sustain marine ecosystem services. Frontiers in Ecology and the Environment 7, 204–211 (2009).
13. Hoegh-Guldberg, O. et al. The ocean as a solution to climate change: Five opportunities for action. (2019).
14. Smale, D. A., Burrows, M. T., Moore, P., O’Connor, N. & Hawkins, S. J. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution 3, 4016–4038 (2013).
15. Ondiviela, B. et al. The role of seagrasses in coastal protection in a changing climate. Coastal Engineering 87, 158–168 (2014).
16. Bloor, I. S. M. et al. Boom not bust: Cooperative management as a mechanism for improving the commercial efficiency and environmental outcomes of regional scallop fisheries. Marine Policy 132, 104649 (2021).
17. Avdelas, L. et al. The decline of mussel aquaculture in the European Union: causes, economic impacts and opportunities. Reviews in Aquaculture 13, 91–118 (2021).
18. Hoegh-Guldberg, O., Northrop, E. & Lubchenco, J. The ocean is key to achieving climate and societal goals. Science 365, 1372–1374 (2019).
19. Laffoley, D. et al. The forgotten ocean: Why COP26 must call for vastly greater ambition and urgency to address ocean change. Aquatic Conservation: Marine and Freshwater Ecosystems 32, 217–228 (2022).
20. Roberts, C. M. et al. Marine reserves can mitigate and promote adaptation to climate change. Proc Natl Acad Sci USA 114, 6167 (2017).
21. Duarte, C. M. et al. Rebuilding marine life. Nature 580, 39–51 (2020).
22. Vad, J. et al. Chapter Two - Potential Impacts of Offshore Oil and Gas Activities on Deep-Sea Sponges and the Habitats They Form. in Advances in Marine Biology (ed. Sheppard, C.) vol. 79 33–60 (Academic Press, 2018).
23. Animah, I. & Shafiee, M. Condition assessment, remaining useful life prediction and life extension decision making for offshore oil and gas assets. Journal of Loss Prevention in the Process Industries 53, 17–28 (2018).
24. Hjorth, M. et al. Effects of Oil and Gas Production On Marine Ecosystems and Fish Stocks in the Danish North Sea: Review. Report for WSP Denmark. https://mst.dk/media/222352/oil_gas-effect-report_final.pdf (2021).
25. Stowe, T. J. & Underwood, L. A. Oil spillages affecting seabirds in the United Kingdom, 1966–1983. Marine Pollution Bulletin 15, 147–152 (1984).
26. Moore, J., Taylor, P. & Hiscock, K. Rocky shores monitoring programme. Proceedings of the Royal Society of Edinburgh. Section B. Biological Sciences 103, 181–200 (1995).
27. Riddick, S. N. et al. Methane emissions from oil and gas platforms in the North Sea. Atmospheric Chemistry and Physics 19, 9787–9796 (2019).
28. Cordes, E. E. et al. Environmental Impacts of the Deep-Water Oil and Gas Industry: A Review to Guide Management Strategies. Frontiers in Environmental Science 4, (2016).
29. Frost, K. J. et al. Alaska, and Adjacent Areas Following the Exxon Valdez Oil Spill Marine Mammal Study Number 5. (1994).
30. Garrott, R. A., Eberhardt, L. L. & Burn, D. M. Mortality of sea otters in Prince William Sound following the Exxon Valdez oil spill. Marine Mammal Science 9, 343–359 (1993).
31. Kerr, R., Kintisch, E. & Stokstad, E. Will Deepwater Horizon Set a New Standard for Catastrophe? Science 328, 674–675 (2010).
32. Montagna, P. A. et al. Deep-Sea Benthic Footprint of the Deepwater Horizon Blowout. PLOS ONE 8, e70540 (2013).
33. Bodkin JL et al. Long-term effects of the Exxon Valdez oil spill: sea otter foraging in the intertidal as a pathway of exposure to lingering oil. Mar Ecol Prog Ser 447, 273–287 (2012).
34. Heintz, R. A., Short, J. W. & Rice, S. D. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon valdez crude oil. Environmental Toxicology and Chemistry 18, 494–503 (1999).
35. Matkin, C. O., Saulitis, E. L., Ellis, G. M., Olesiuk, P. & Rice, S. D. Ongoing population-level impacts on killer whales Orcinus orca following the ‘Exxon Valdez’ oil spill in Prince William Sound, Alaska. Marine Ecology Progress Series 356, 269–281 (2008).
36. Muehlenbachs, L., Cohen, M. A. & Gerarden, T. The impact of water depth on safety and environmental performance in offshore oil and gas production. Energy Policy 55, 699–705 (2013).
37. Gallego, A. et al. Current status of deepwater oil spill modelling in the Faroe-Shetland Channel, Northeast Atlantic, and future challenges. Marine Pollution Bulletin 127, 484–504 (2018).
38. Jernelov, A. The threats from oil spills: now, then, and in the future. Ambio 39, 353–366 (2010).
39. Moore, J. et al. SEA EMPRESS SPILL: IMPACTS ON MARINE AND COASTAL HABITATS. International Oil Spill Conference Proceedings 1997, 213–216 (1997).
40. Banks, A. N. et al. The Sea Empress oil spill (Wales, UK): Effects on Common Scoter Melanitta nigra in Carmarthen Bay and status ten years later. Marine Pollution Bulletin 56, 895–902 (2008).
41. Webster, L. et al. Long-term Monitoring of Polycyclic Aromatic Hydrocarbons in Mussels (Mytilus edulis) Following the Braer Oil Spill†. Analyst 122, 1491–1495 (1997).
42. Conroy, J. & Kruuk, H. Changes in Otter Numbers in Shetland Between 1988 and 1993. Oryx vol. 29 197–204 (1995).
43. Hall, A. J., Watkins, J. & Hiby, L. The impact of the 1993 Braer oil spill on grey seals in Shetland. Science of The Total Environment 186, 119–125 (1996).
44. Goodlad, J. Effects of the Braer oil spill on the Shetland seafood industry. Science of The Total Environment 186, 127–133 (1996).
45. Equinor. Rosebank Environmental Statement ES/2022/001.
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1097880/Rosebank_Environmental_Statement_-_Final_for_Submission_To_OPRED_Equinor_3rd_August_2022.pdf (2022).
46. Baker, J. R., Jones, A. M., Jones, T. P. & Watson, H. C. Otter Lutra lutra L. mortality and marine oil pollution. Biological Conservation 20, 311–321 (1981).
47. Joye, S. B. et al. The Gulf of Mexico ecosystem, six years after the Macondo oil well blowout. Deep Sea Research Part II: Topical Studies in Oceanography 129, 4–19 (2016).
48. Law, R. J. & Hellou, J. Contamination of Fish and Shellfish Following Oil Spill Incidents. Environmental Geosciences 6, 90–98 (1999).
49. Bakhmet, I., Fokina, N. & Ruokolainen, T. Changes of Heart Rate and Lipid Composition in Mytilus Edulis and Modiolus Modiolus Caused by Crude Oil Pollution and Low Salinity Effects. J Xenobiot 11, 46–60 (2021).
50. Farinas-Franco, J. M. et al. Are we there yet? Management baselines and biodiversity indicators for the protection and restoration of subtidal bivalve shellfish habitats. Science of The Total Environment 863, 161001 (2023).
51. DeLeo, D. M., Ruiz-Ramos, D. V., Baums, I. B. & Cordes, E. E. Response of deep-water corals to oil and chemical dispersant exposure. Deep Sea Research Part II: Topical Studies in Oceanography 129, 137–147 (2016).
52. DeLeo, D. M., Lengyel, S. D. & Cordes, E. E. Assessing Oil Spill Impacts to Cold-Water Corals of the Deep Gulf of Mexico. 2016, PO13F-05 (2016).
53. OSPAR. Assessment of the OSPAR Report on Discharges, Spills and Emissions from Offshore Installations 2009 – 2018.
54. OSPAR. OSPAR report on discharges, spills and emissions from offshore oil and gas installations in 2012.
55. Dong, Y., Liu, Y., Hu, C., MacDonald, I. R. & Lu, Y. Chronic oiling in global oceans. Science 376, 1300–1304 (2022).
56. Camphuysen, K. C. J. Declines in oil-rates of stranded birds in the North Sea highlight spatial patterns in reductions of chronic oil pollution. Marine Pollution Bulletin 60, 1299–1306 (2010).
57. Camphuysen, K. & Heubeck, M. Beached Bird Surveys in the North Sea as an Instrument to Measure Levels of Chronic Oil Pollution. In Oil Pollution in the North Sea (ed. Carpenter, A.) 193–208 (Springer International Publishing, 2016). doi:10.1007/698_2015_435.
58. Neff, J., Lee, K. & DeBlois, E. M. Produced Water: Overview of Composition, Fates, and Effects. in Produced Water: Environmental Risks and Advances in Mitigation Technologies (eds.Lee, K. & Neff, J.) 3–54 (Springer New York, 2011). doi:10.1007/978-1-4614-0046-2_1.
59. OSPAR. Report on impacts of discharges of oil and chemicals in produced water on the marine environment. https://www.ospar.org/documents?v=47303 (2021).
60. Bakke, T., Klungsoyr, J. & Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Marine Environmental Research 92, 154–169 (2013).
61. Hansen, B. H. et al. Embryonic exposure to produced water can cause cardiac toxicity and deformations in Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) larvae. Marine Environmental Research 148, 81–86 (2019).
62. Meier, S. et al. DNA damage and health effects in juvenile haddock (Melanogrammus aeglefinus) exposed to PAHs associated with oil-polluted sediment or produced water. PLOS ONE 15, e0240307 (2020).
63. Neff, J. M. Polycyclic aromatic hydrocarbons in the aquatic environment. Biol. Conserv.;(United Kingdom) 18, (1980).
64. Tuvikene, A. Responses of fish to polycyclic aromatic hydrocarbons (PAHs). Annales Zoologici Fennici 32, 295–309 (1995).
65. Sundt, R. C., Pampanin, D. M., Grung, M., Baršienė, J. & Ruus, A. PAH body burden and biomarker responses in mussels (Mytilus edulis) exposed to produced water from a North Sea oil field: Laboratory and field assessments. Marine Pollution Bulletin 62, 1498–1505 (2011).
66. Capuzzo, J. M., Lancaster, B. A. & Sasaki, G. C. The effects of petroleum hydrocarbons on lipid metabolism and energetics of larval development and metamorphosis in the american lobster (homarus americanus Milne Edwards). Marine Environmental Research 14, 201–228 (1984).
67. Kho, F. et al. Current understanding of the ecological risk of mercury from subsea oil and gas infrastructure to marine ecosystems. Journal of Hazardous Materials 438, 129348 (2022).
68. Al-Kindi, S., Al-Bahry, S., Al-Wahaibi, Y., Taura, U. & Joshi, S. Partially hydrolyzed polyacrylamide: enhanced oil recovery applications, oil-field produced water pollution, and possible solutions. Environmental Monitoring and Assessment 194, 875 (2022).
69. Xiong, B. et al. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 1, 1–9 (2018).
70. Cruz, A. M. & Krausmann, E. Vulnerability of the oil and gas sector to climate change and extreme weather events. Climatic Change 121, 41–53 (2013).
71. Santos, M. F. L., Lana, P. C., Silva, J., Fachel, J. G. & Pulgati, F. H. Effects of non-aqueous fluids cuttings discharge from exploratory drilling activities on the deep-sea macrobenthic communities. Deep Sea Research Part II: Topical Studies in Oceanography 56, 32–40 (2009).
72. Veltman, K., Huijbregts, M. A., Rye, H. & Hertwich, E. G. Including impacts of particulate emissions on marine ecosystems in life cycle assessment: The case of offshore oil and gas production. Integrated Environmental Assessment and Management 7, 678–686 (2011).
73. Taylor, J., Drewery, J., & Boulcott, P. 1218S Cruise Report: Monitoring survey of Faroe-Shetland Sponge Belt NCMPA, Rosemary Bank Seamount NCMPA and Wyville Thomson Ridge SAC, (2019).
74. Jones DOB, Gates AR, & Lausen B. Recovery of deep-water megafaunal assemblages from hydrocarbon drilling disturbance in the Faroe−Shetland Channel. Mar Ecol Prog Ser 461, 71–82 (2012).
75. Duarte, C. M. et al. The soundscape of the Anthropocene ocean. Science 371, eaba4658 (2021).
76. Krause, B. Voices of the wild: animal songs, human din, and the call to save natural soundscapes. (Yale University Press, 2015).
77. Williams, W.J., Curnick, D.J., & Deaville, R. (last). Identification of key species in the UK, with a focus on English waters, sensitive to underwater noise, (2021).
78. Chou, E., Southall, B. L., Robards, M. & Rosenbaum, H. C. International policy, recommendations, actions and mitigation efforts of anthropogenic underwater noise. Ocean & Coastal Management 202, 105427 (2021).
79. Rako-Gospi, N. & Picciulin, M. Chapter 20 - Underwater Noise: Sources and Effects on Marine Life. in World Seas: An Environmental Evaluation (Second Edition) (ed. Sheppard, C.) 367–389 (Academic Press, 2019).
80. Mooney, T. A., Andersson, M. H. & Stanley, J. Acoustic impacts of offshore wind energy on fishery resources. Oceanography 33, 82–95 (2020).
81. Nowacek, D. P. et al. Marine seismic surveys and ocean noise: time for coordinated and prudent planning. Frontiers in Ecology and the Environment 13, 378–386 (2015).
82. Erbe, C., Duncan, A., Hawkins, L., Terhune, J. M. & Thomas, J. A. Introduction to Acoustic Terminology and Signal Processing. in Exploring Animal Behavior Through Sound: Volume 1 111–152 (Springer, 2022).
83. Nieukirk, S. L. et al. Sounds from airguns and fin whales recorded in the mid-Atlantic Ocean, 1999–2009. The Journal of the Acoustical Society of America 131, 1102–1112 (2012).
84. McCauley, R. D. et al. Widely used marine seismic survey air gun operations negatively impact zooplankton. Nat Ecol Evol 1, 1–8 (2017).
85. Mann, D. et al. Hearing Loss in Stranded Odontocete Dolphins and Whales. PLOS ONE 5, e13824 (2010).
86. Kavanagh, A. S., Nykanen, M., Hunt, W., Richardson, N. & Jessopp, M. J. Seismic surveys reduce cetacean sightings across a large marine ecosystem. Sci Rep 9, 19164 (2019).
87. Castellote, M. & Llorens, C. Review of the Effects of Offshore Seismic Surveys in Cetaceans: Are Mass Strandings a Possibility? in The Effects of Noise on Aquatic Life II (eds. Popper, A. N. & Hawkins, A.) 133–143 (Springer New York, 2016).
88. Lucke, K., Siebert, U., Lepper, P. A. & Blanchet, M.-A. Temporary shift in masked hearing thresholds in a harbor porpoise (Phocoena phocoena) after exposure to seismic airgun stimuli. The Journal of the Acoustical Society of America 125, 4060–4070 (2009).
89. Pirotta, E., Brookes, K. L., Graham, I. M. & Thompson, P. M. Variation in harbour porpoise activity in response to seismic survey noise. Biology Letters 10, 20131090 (2014).
90. Gordon, J. C. D. et al. A Review of the Effects of Seismic Survey on Marine Mammals. Marine Technology Society Journal 37, (2003).
91. Stone, C. J. The effects of seismic activity on marine mammals in UK waters, 1998-2000, (2003).
92. Stone, C. J., Hall, K., Mendes, S. & Tasker, M. L. The effects of seismic operations in UK waters: analysis of Marine Mammal Observer data. Journal of Cetacean Research and Management (2017).
93. Dunlop, R. A. et al. The behavioural response of migrating humpback whales to a full seismic airgun array. Proceedings of the Royal Society B: Biological Sciences 284, 20171901 (2017).
94. McCauley, R. D. et al. MARINE SEISMIC SURVEYS— A STUDY OF ENVIRONMENTAL IMPLICATIONS. The APPEA Journal 40, 692–708 (2000).
95. Wisniewska, D. M. et al. Ultra-High Foraging Rates of Harbor Porpoises Make Them Vulnerable to Anthropogenic Disturbance. Current Biology 26, 1441–1446 (2016).
96. Nelms, S. E., Piniak, W. E. D., Weir, C. R. & Godley, B. J. Seismic surveys and marine turtles: An underestimated global threat? Biological Conservation 193, 49–65 (2016).
97. Soto, N. A. et al. Anthropogenic noise causes body malformations and delays development in marine larvae. Sci Rep 3, 1–5 (2013).
98. Guerra, A., Gonzalez, A. & Rocha, F. A review of records of giant squid in the north-eastern Atlantic and severe injuries in Architeuthis dux stranded after acoustic exploration. ICES CM 2004, (2004).
99. Sole, M., Monge, M., Andre, M. & Quero, C. A proteomic analysis of the statocyst endolymph in common cuttlefish (Sepia officinalis): an assessment of acoustic trauma after exposure to sound. Scientific Reports 9, 9340 (2019).
100. Day, R. D., McCauley, R. D., Fitzgibbon, Q. P., Hartmann, K. & Semmens, J. M. Seismic air guns damage rock lobster mechanosensory organs and impair righting reflex. Proceedings of the Royal Society B: Biological Sciences 286, 20191424 (2019).
101. Andre, M. et al. Low-frequency sounds induce acoustic trauma in cephalopods. Frontiers in Ecology and the Environment 9, 489–493 (2011).
102. Davidsen, J. G. et al. Effects of sound exposure from a seismic airgun on heart rate, acceleration and depth use in free-swimming Atlantic cod and saithe. Conservation Physiology 7, coz020 (2019).
103. Sierra-Flores, R., Atack, T., Migaud, H. & Davie, A. Stress response to anthropogenic noise in Atlantic cod Gadus morhua L. Aquacultural Engineering 67, 67–76 (2015).
104. Engas, A. & Lokkeborg, S. Effects of seismic shooting and vessel-generated noise on fish behaviour and catch rates. Bioacoustics 12, 313–316 (2002).
105. Lokkeborg, S., Ona, E., Vold, A. & Salthaug, A. Sounds from seismic air guns: gear- and species-specific effects on catch rates and fish distribution. Can. J. Fish. Aquat. Sci. 69, 1278–1291 (2012).
106. Slotte, A., Hansen, K., Dalen, J. & Ona, E. Acoustic mapping of pelagic fish distribution and abundance in relation to a seismic shooting area off the Norwegian west coast. Fisheries Research 67, 143–150 (2004).
107. Hassel, A. et al. Reaction of sand eel to seismic shooting: A field experiment and fishery statistics study. (2003).
108. Hassel, A. et al. Influence of seismic shooting on the lesser sandeel (Ammodytes marinus). ICES Journal of Marine Science - ICES J MAR SCI 61, 1165–1173 (2004).
109. Merchant, N. D. & Robinson, S. Abatement of underwater noise pollution from pile-driving and explosions in UK waters. in vol. 12 (2019).
110. Dahne, M. et al. Effects of pile-driving on harbour porpoises (Phocoena phocoena) at the first offshore wind farm in Germany. Environmental Research Letters 8, 025002 (2013).
111. Genesis Oil and Gas & Consultants. Review and Assessment of Underwater Sound Produced from Oil and Gas Sound Activities and Potential Reporting Requirements under the Marine Strategy Framework Directive, (2011).
112. Fowler, A. M. et al. Environmental benefits of leaving offshore infrastructure in the ocean.Frontiers in Ecology and the Environment 16, 571–578 (2018).
113. MacIntosh, A., Dafforn, K., Penrose, B., Chariton, A. & Cresswell, T. Ecotoxicological effects of decommissioning offshore petroleum infrastructure: A systematic review. Critical Reviews in Environmental Science and Technology 52, 3283–3321 (2022).
114. Cantle, P. & Bernstein, B. Air emissions associated with decommissioning California’s offshore oil and gas platforms. Integrated Environmental Assessment and Management 11, 564–571 (2015).
115. Coolen, J. W. P. et al. Ecological implications of removing a concrete gas platform in the North Sea. Journal of Sea Research 166, 101968 (2020).
116. Stap, T., Coolen, J. W. P. & Lindeboom, H. J. Marine Fouling Assemblages on Offshore Gas Platforms in the Southern North Sea: Effects of Depth and Distance from Shore on Biodiversity. PLOS ONE 11, e0146324 (2016).
117. Ahiaga-Dagbui, D., Whyte, A. & Boateng, P. Costing and Technological Challenges of Offshore Oil and Gas Decommissioning in the UK North Sea. Journal of Construction Engineering and Management 143, (2017).
118. Jorgensen, D. OSPAR’s exclusion of rigs-toreefs in the North Sea. Ocean & Coastal Management 58, 57–61 (2012).
119. OSPAR. OSPAR Decision 98/3 on the Disposal of Disused Offshore Installations, (1998).
120. Fortune, I. S. & Paterson, D. M. Ecological best practice in decommissioning: a review of scientific research. ICES Journal of Marine Science 77, 1079–1091 (2020).
121. Tidbury, H. et al. Social network analysis as a tool for marine spatial planning: Impacts of decommissioning on connectivity in the North Sea. Journal of Applied Ecology 57, 566–577 (2020).
122. Ekins, P., Vanner, R. & Firebrace, J. Decommissioning of offshore oil and gas facilities: A comparative assessment of different scenarios. Journal of Environmental Management 79, 420–438 (2006).
123. Aslani, F., Zhang, Y., Manning, D., Valdez, L. C. & Manning, N. Additive and alternative materials to cement for well plugging and abandonment: A state-of-the-art review. Journal of PetroleumScience and Engineering 215, 110728 (2022).
124. North Sea Transition Authority (NSTA). UKCS Decommissioning Cost Estimate 2022, (2022).
125. Hegmann, G. et al. Cumulative Effects Assessment Practitioners Guide. (1999).
126. Kirkfeldt, T. S. et al. Why cumulative impacts assessments of hydrocarbon activities in the Arctic fail to meet their purpose. Reg Environ Change 17, 725–737 (2017).
127. Pirotta, E. et al. Understanding the combined effects of multiple stressors: A new perspective on a longstanding challenge. Science of The Total Environment 821, 153322 (2022).
128. Bindoff, N. L. et al. Changing ocean, marine ecosystems, and dependent communities. IPCC special report on the ocean and cryosphere in a changing climate 477–587 (2019).
129. Gissi, E. et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Science of The Total Environment 755, 142564 (2021).
130. Jackson, E. L., Davies, A. J., Howell, K. L., Kershaw, P. J. & Hall-Spencer, J. M. Future-proofing marine protected area networks for cold water coral reefs. ICES Journal of Marine Science 71, 2621–2629 (2014).
131. Roberts, D. A. et al. Ocean acidification increases the toxicity of contaminated sediments. Global Change Biology 19, 340–351 (2013). 132. Willsteed, E., Gill, A. B., Birchenough, S. N. R. & Jude, S. Assessing the cumulative environmental effects of marine renewable energy developments: Establishing common ground. Science of The Total Environment 577, 19–32 (2017).
133. Laffoley, D. et al. Chapter 29 – Marine Protected Areas. in World Seas: An Environmental Evaluation (Second Edition) (ed. Sheppard, C.) 549–569 (Academic Press, 2019).
134. Roberts, C. M., Bohnsack, J. A., Gell, F., Hawkins, J. P. & Goodridge, R. Effects of Marine Reserves on Adjacent Fisheries. Science 294, 1920–1923 (2001).
135. Ban, N. C. et al. Well-being outcomes of marine protected areas. Nature Sustainability 2, 524–532 (2019).
136. Sheehan, E. V. et al. Rewilding of Protected Areas Enhances Resilience of Marine Ecosystems to Extreme Climatic Events. Frontiers in Marine Science 8, (2021).
137. Gell, F. R. & Roberts, C. M. Benefits beyond boundaries: the fishery effects of marine reserves. Trends in Ecology & Evolution 18, 448–455 (2003).
138. Convention on Biological Diversity. Final text of Kunming-Montreal Global Biodiversity Framework. (2022).
139. JNCC. UK Marine Protected Area Network Statistics, (2022).
140. North Sea Transition Authority (NSTA), Offshore Petroleum Licensing Rounds,(2022).
141. Guidelines for applying the IUCN protected area management categories to marine protected areas. (IUCN).
142. Stolton, S., Shadie, P., & Dudley, N. IUCN WCPA Best Practice Guidance on Recognising Protected Areas and Assigning Management Categories and Governance Types.
143. Burdon, D., Barnard, S., Boyes, S. J. & Elliott, M. Oil and gas infrastructure decommissioning in marine protected areas: System complexity, analysis and challenges. Marine Pollution Bulletin 135, 739–758 (2018).
144. Marine Management Organisation. The Dogger Bank Special Area of Conservation (Specified Area) Bottom Towed Fishing Gear Byelaw 2022, (2022).
145. JNCC. English Highly Protected Marine Areas. (2022).
146. Scottish Government. Highly Protected Marine Areas (HPMAs) - site selection: draft guidelines. / (2022).
147. Marine Conservation Society & Rewilding Britain. Marine Conservation Society & Rewilding Britain. (2022) Blue carbon: Ocean-based solutions to fight the climate crisis. A report by the Marine Conservation Society and Rewilding Britain. (2022).
148. Grorud-Colvert, K. et al. The MPA Guide: A framework to achieve global goals for the ocean. Science 373, eabf0861 (2021).
149. Santo, E. M. D. Assessing public participation in environmental decision-making: Lessons learned from the UK Marine Conservation Zone (MCZ) site selection process. Marine Policy 64, 91–101 (2016).
150. Engel, M. T. & Vaske, J. J. Balancing public acceptability and consensus regarding marine protected areas management using the Potential for Conflict Index2. Marine Policy 139, 105042 (2022).
151. Gies, Erica. Canada Has New Rules Governing Its Marine Protected Areas. Do They Go Far Enough? (2019).
152. DEFRA. Inner Silver Pit South: Consultation factsheet for candidate Highly Protected Marine Area (HPMA). (2022).
153. Gamble, C. et al. Seagrass Restoration Handbook: UK and Ireland. in (Zoological Society of London, 2021).
154. Fodrie, F. J. et al. Oyster reefs as carbon sources and sinks. Proceedings of the Royal Society B: Biological Sciences 284, 20170891 (2017).
155. Green, A. E., Unsworth, R. K. F., Chadwick, M. A. & Jones, P. J. S. Historical Analysis Exposes Catastrophic Seagrass Loss for the United Kingdom. Frontiers in Plant Science 12, (2021).
156. Lovelock, C. E. & Reef, R. Variable Impacts of Climate Change on Blue Carbon. One Earth 3, 195–211 (2020).
157. Climate Change Committee. Briefing: Blue Carbon-March 2022 Climate Change Committee. (2022).
158. Costa, M. & Macreadie, P. The Evolution of Blue Carbon Science. Wetlands 42, (2022).
159. Claes, J., Hopman, D., Jaeger, G. & Rogers, M. Blue carbon: The potential of coastal and oceanic climate action. (2022).
160. Macreadie, P. I. et al. Blue carbon as a natural climate solution. Nature Reviews Earth & Environment 2, 826–839 (2021).
161. Burden, Annette & Clilverd, Hannah. Moving towards inclusion of coastal wetlands in the UK LULUCF inventory: rapid assessment of activity data availability. 61 (2022).
162. Krause-Jensen, D. et al. Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biology Letters 14, 20180236 (2018).
163. Cullen-Unsworth, L. C. & Unsworth, R. K. Strategies to enhance the resilience of the world’s seagrass meadows. Journal of Applied Ecology 53, 967–972 (2016).
164. Hiraishi, T. et al. 2013 supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: Wetlands. IPCC, Switzerland (2014).
165. Austin, W., Smeaton, C., Houston, A. & Balke, T. Scottish saltmarsh, sea-level rise, and the potential for managed realignment to deliver blue carbon gains. (2022).
166. Smeaton, C. et al. Using citizen science to estimate surficial soil Blue Carbon stocks in Great British saltmarshes. Frontiers in Marine Science 461 (2022).
167. Boorman, L. Saltmarsh Review. An overview of coastal saltmarshes, their dynamic and sensitivity characteristics for conservation and management. (2003).
168. Davy, A., Bakker, J. & Figueroa, M. Human modification of European salt marshes. Human impacts on salt marshes: a global perspective. University of California Press, Berkeley, California, USA 311–336 (2009).
169. Jones, B. L. & Unsworth, R. K. F. The perilous state of seagrass in the British Isles. Royal Society Open Science 3, 150596.
170. Arias-Ortiz, A. et al. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nature Climate Change 8, 338–344 (2018).
171. Porter, J. et al. Blue carbon audit of Orkney waters. (2020).
172. Austin, W., Turrell, W. & Tilbrook, C. A brief history of Scottish blue carbon science and the Scottish Blue Carbon Forum: Where next? in.
173. Burrows, M. et al. Assessment of carbon capture and storage in natural systems within the English North Sea (Including within Marine Protected Areas). (2021).
174. Mariani, G. et al. Let more big fish sink: Fisheries prevent blue carbon sequestration—half in unprofitable areas. Science Advances 6, eabb4848.
175. Pershing, A. J., Christensen, L. B., Record, N. R., Sherwood, G. D. & Stetson, P. B. The Impact of Whaling on the Ocean Carbon Cycle: Why Bigger Was Better. PLOS ONE 5, e12444 (2010).
176. Christianson, A. B. et al. The promise of blue carbon climate solutions: where the science supports ocean-climate policy. Frontiers in Marine Science 589 (2022).
177. Durfort, A. et al. Recovery of carbon benefits by overharvested baleen whale populations is threatened by climate change. Proceedings of the Royal Society B: Biological Sciences 289, 20220375 (2022).
178. Tulloch, V. J. D., Plaganyi, E. E., Brown, C., Richardson, A. J. & Matear, R. Future recovery of baleen whales is imperiled by climate change. Global Change Biology 25, 1263–1281 (2019).
179. Bijma, J., Portner, H.-O., Yesson, C. & Rogers, A. D. Climate change and the oceans – What does the future hold? Marine Pollution Bulletin 74, 495–505 (2013).
180. Wright, P., Pinnegar, J. & Fox, C. Impacts of climate change on fish, relevant to the coastal and marine environment around the UK. 354–381 (2020).
181. Gormley, K. et al. Connectivity and Dispersal Patterns of Protected Biogenic Reefs: Implications for the Conservation of Modiolus modiolus (L.) in the Irish Sea. PLOS ONE 10, e0143337 (2015).
182. Hiscock, K. Exploring Britain’s Hidden World: A Natural History of Seabed Habitats. (2018).
183. Edwards, M. et al. Plankton, jellyfish and climate in the North-East Atlantic. MCCIP Sci. Rev 2020, 322–353 (2020).
184. Fromentin JM & Planque B. Calanus and environment in the eastern North Atlantic. II. Influence of the North Atlantic Oscillation on C. finmarchicus and C. helgolandicus. Mar Ecol Prog Ser 134, 111–118 (1996).
185. Engelhard, G. H., Righton, D. A. & Pinnegar, J. K. Climate change and fishing: a century of shifting distribution in North Sea cod. Global Change Biology 20, 2473–2483 (2014).
186. Evans, P. G. H. & Waggitt, J. J. Impacts of climate change on marine mammals, relevant to the coastal and marine environment around the UK (MCCIP Science Review 2020).
187. Vezzulli, L. et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proceedings of the National Academy of Sciences 113, E5062–E5071 (2016).
188. Walsh, J. E. et al. The high latitude marine heat wave of 2016 and its impacts on Alaska. Bull. Am. Meteorol. Soc 99, S39–S43 (2018).
189. IPCC. Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. 151 (2014).
190. Mahaffey, C., Palmer, M., Greenwood, N. & Sharples, J. Impacts of climate change on dissolved oxygen concentration relevant to the coastal and marine environment around the UK. MCCIP Science Review 31–53 (2020).
191. Smith, K. E. et al. Biological Impacts of Marine Heatwaves. Annu. Rev. Mar. Sci. (2023).
192. Oliver, E. C. J. et al. Marine Heatwaves. Annu.Rev. Mar. Sci. 13, 313–342 (2021).
193. Bell, J. J. et al. Marine heat waves drive bleaching and necrosis of temperate sponges. Current Biology 33, 158-163.e2 (2023).
194. Leon, P. et al. Relationship between shell integrity of pelagic gastropods and carbonate chemistry parameters at a Scottish Coastal Observatory monitoring site. ICES Journal of Marine Science 77, 436–450 (2020).
195. Findlay, H. et al. Climate change impacts on ocean acidification relevant to the UK and Ireland. MCCIP Science Review 2 (2022).
196. Wolf, J., Woolf, D. & Bricheno, L. Impacts of climate change on storms and waves relevant to the coastal and marine environment around the UK. MCCIP Science Review 2020, 132–157 (2020).
197. Vanem, E. & Bitner-Gregersen, E. M. Stochastic modelling of long-term trends in the wave climate and its potential impact on ship structural loads. Applied Ocean Research 37, 235–248 (2012).
198. Bitner-Gregersen, E. M., Eide, L. I., Horte, T. & Skjong, R. Potential Impact of Climate Change on Design of Ship and Offshore Structures. in Ship and Offshore Structure Design in Climate Change Perspective 43–52 (Springer Berlin Heidelberg, 2013).
199. Thompson, R. C. et al. Lost at Sea: Where Is All the Plastic? Science 304, 838–838 (2004).
200. Stafford, R. & Jones, P. J. S. Viewpoint – Ocean plastic pollution: A convenient but distracting truth? Marine Policy 103, 187–191 (2019).
201. Ford, H. V. et al. The fundamental links between climate change and marine plastic pollution. Science of The Total Environment 806, 150392 (2022).
202. Bergmann, M. et al. Plastic pollution in the Arctic. Nat Rev Earth Environ 3, 323–337 (2022).
203. Wyllie, J. Oil industry dumping tons of microplastics into North Sea each year. Press and Journal (2018).
204. Knutsen, H. et al. Microplastic accumulation by tube-dwelling, suspension feeding polychaetes from the sediment surface: A case study from the Norwegian Continental Shelf. Marine Environmental Research 161, 105073 (2020).
205. La Beur, L. et al. Baseline Assessment of Marine Litter and Microplastic Ingestion by Cold-Water Coral Reef Benthos at the East Mingulay Marine Protected Area (Sea of the Hebrides, Western Scotland). Frontiers in Marine Science 6, (2019).
206. Wright, S. L., Rowe, D., Thompson, R. C. & Galloway, T. S. Microplastic ingestion decreases energy reserves in marine worms. Current Biology 23, R1031–R1033 (2013).
207. Roman, L., Schuyler, Q., Wilcox, C. & Hardesty, B. D. Plastic pollution is killing marine megafauna, but how do we prioritize policies to reduce mortality? Conservation Letters 14, e12781 (2021).
208. Adyel, T. M. & Macreadie, P. I. Plastics in blue carbon ecosystems: a call for global cooperation on climate change goals. The Lancet Planetary Health 6, e2–e3 (2022).
209. Smith, M., Love, D. C., Rochman, C. M. & Neff, R. A. Microplastics in Seafood and the Implications for Human Health. Current Environmental Health Reports 5, 375–386 (2018).
210. Bauer, F. & Fontenit, G. Plastic dinosaurs – Digging deep into the accelerating carbon lock-in of plastics. Energy Policy 156, 112418 (2021).
211. Carpenter, S. Why The Oil Industry’s $400 Billion Bet On Plastics Could Backfire. Forbes (2020).
212. Lo, J. Plastics resolution tees up battle over oil industry’s plan B, Climate Home News. Climate Home News (2022).
213. Oceana. The Cost of Amazon’s Plastic Denial on the World’s Oceans. (2022).
214. BP. BP Energy Outlook – 2018 edition. (2018).
215. Shell. 4 Segment information - Shell Annual Report 2020. (2021).
216. International Energy Agency. The Future of Petrochemicals, (2018).
217. BEIS. UK Offshore Energy Strategic Environmental Assessment 4 (OESEA4).
218. CIEEM. The Guidelines for Ecological Impact Assessment in the UK and Ireland. (2018).
219. Barker, A. & Jones, C. A critique of the performance of EIA within the offshore oil and gas sector. Environmental Impact Assessment Review 43, 31–39 (2013).
220. Hancox, E. QUALITY REVIEW OF OFFSHORE PETROLEUM DEVELOPMENT ENVIRONMENTAL STATEMENTS AGAINST THE EIA DIRECTIVE. 50 (2019).
221. Clark, M. R., Durden, J. M. & Christiansen, S. Environmental Impact Assessments for deep-sea mining: Can we improve their future effectiveness? Marine Policy 114, (2020).
222. OSPAR. Status Assessment 2022 - Deep-sea sponge aggregations. (2022).
223. OPRED. Environmental Statement Summary - Alligin Development.(2018).
224. BP. BP announces first oil from Alligin field, west of Shetland | News and insights | Home.
225. BEIS & OPRED. Offshore Energy Strategic Environmental Assessment (SEA): An overview of the SEA process. (2023).
226. Currie, D. R. & Isaacs, L. R. Impact of exploratory offshore drilling on benthic communities in the Minerva gas field, Port Campbell, Australia. Marine Environmental Research 59, 217–233 (2004).
227. Ellis, J., Fraser, G. & J, R. Discharged drilling waste from oil and gas platforms and its effects on benthic communities. Marine Ecology Progress Series 456, 285–302 (2012).
228. Gates, A. R. & Jones, D. O. B. Recovery of Benthic Megafauna from Anthropogenic Disturbance at a Hydrocarbon Drilling Well (380 m Depth in the Norwegian Sea). PLOS ONE 7, e44114 (2012).
229. Stein J E, Reichert W L, & Varanasi U. Molecular epizootiology: assessment of exposure to genotoxic compounds in teleosts. Environmental Health Perspectives 102, 19–23 (1994).
230. Gerrard, S., Grant, A., Marsh, R. & London, C. Drill cuttings piles in the North Sea: management options during platform decommissioning. Centre for Environmental Risk, Res. Rpt (1999).
231. Helm, R. C. et al. Overview of effects of oil spills on marine mammals. Handbook of oil spill science and technology 455–475 (2014).
232. Venn-Watson, S. et al. Adrenal Gland and Lung Lesions in Gulf of Mexico Common Bottlenose Dolphins (Tursiops truncatus) Found Dead following the Deepwater Horizon Oil Spill. PLOS ONE 10, e0126538 (2015). 233. OSPAR. Precautionary Principle. (2022).
234. Beyond Oil and Gas Alliance (2022). (2022).
235. Linde, L., Sanchez, F., Mete, G. & Lindberg, A. North Sea oil and gas transition from a regional and global perspective. (2022).
236. NSW Government. NSW Government rules out commercial offshore exploration and mining. (2022).
237. Government of Canada. Protection Standardsto better conserve our oceans. (2022).
238. Kapoor, A., Fraser, G. S. & Carter, A. Marine conservation versus offshore oil and gas extraction: Reconciling an intensifying dilemma in Atlantic Canada. The Extractive Industries and Society 8, 100978 (2021).
239. Fisheries and Oceans Canada. Backgrounder: Laurentian Channel Marine Protected Area. (2019).
240. Office of National Marine Sanctuaries. Regulations. (No date).
241. Papahānaumokuākea Marine National Monument. Papahānaumokuākea Marine National Monument Permitting. (No date).
242. Great Barrier Reef Marine Park Authority. Activities and use. (2022).
243. Jaspars, M. et al. The marine biodiscovery pipeline and ocean medicines of tomorrow. Journal of the Marine Biological Association of the United Kingdom 96, 151–158 (2016).
244. Hyder, K., Maravelias, C. D., Kraan, M., Radford, Z. & Prellezo, R. Marine recreational fisheries — current state and future opportunities. ICES Journal of Marine Science 77, 2171–2180 (2020).
245. White, M. P., Elliott, L. R., Gascon, M., Roberts, B. & Fleming, L. E. Blue space, health and well-being: A narrative overview and synthesis of potential benefits. Environmental Research 191, 110169 (2020).
246. Venegas-Li, R. et al. Global assessment of marine biodiversity potentially threatened by offshore hydrocarbon activities. Global Change Biology 25, 2009–2020 (2019).
247. Gattuso, J.-P. et al. Ocean solutions to address climate change and its effects on marine ecosystems. Frontiers in Marine Science 337 (2018).
248. IAMMWG, C. C. & Siemensma, M. A Conservation Literature Review for the Harbour Porpoise (Phocoena phocoena). JNCC Report (2015).
249. Evans, P. & Waggitt, J. Impacts of climate change on Marine Mammals, relevant to the coastal and marine environment around the UK. (2020).
250. HELCOM. Red List of Baltic Sea underwater biotopes, habitats and biotope complexes. (2013).
251. Borrell, A. PCB and DDT in blubber of cetaceans from the northeastern north Atlantic. Marine Pollution Bulletin 26, 146–151 (1993).
252. Law, R. J. & Whinnett, J. A. Polycyclic aromatic hydrocarbons in muscle tissue of harbour porpoises (Phocoena phocoena) from UK waters. Marine Pollution Bulletin 24, 550–553 (1992).
253. Heuvel-Greve, M. J. van den et al. Polluted porpoises: Generational transfer of organic contaminants in harbour porpoises from the southern North Sea. Science of The Total Environment 796, 148936 (2021).
254. Graham, I. M. et al. Harbour porpoise responses to pile-driving diminish over time. Royal Society Open Science 6, 190335 (2019).
255. Todd, V. L., Williamson, L. D., Couto, A. S., Todd, I. B. & Clapham, P. J. Effect of a new offshore gas platform on harbor porpoises in the Dogger Bank. Marine Mammal Science 38, 1609–1622 (2022).
256. Jarvela Rosenberger, A. L., MacDuffee, M., Rosenberger, A. G. J. & Ross, P. S. Oil Spills and Marine Mammals in British Columbia, Canada: Development and Application of a Risk-Based Conceptual Framework. Archives of Environmental Contamination and Toxicology 73, 131–153 (2017).
257. Helm, R. C. et al. Overview of Effects of Oil Spills on Marine Mammals. in Handbook of Oil Spill Science and Technology 455–475 (2014).
258. Williams, R. et al. Levels of polychlorinated biphenyls are still associated with toxic effects in harbor porpoises (Phocoena phocoena) despite having fallen below proposed toxicity thresholds. Environmental Science & Technology 54, 2277–2286 (2020).
259. Santos, M. & Pierce, G. The diet of harbour porpoise (Phocoena phocoena) in the Northeast Atlantic. Oceanogr Mar Biol Annu Rev 41, 355–390 (2003).
260. Hogg, M. et al. Deep-sea sponge grounds: reservoirs of biodiversity. UNEP-WCMC biodiversity series 32, 1–86 (2010).
261. Lea-Anne Henry & Roberts, J. Applying the OSPAR habitat definitions of deep-sea sponge aggregations to verify suspected records of the habitat in UK waters. 508 (2014).
262. Cathalot, C. et al. Cold-water coral reefs and adjacent sponge grounds: hotspots of benthic respiration and organic carbon cycling in the deep sea. Frontiers in Marine Science 2, (2015).
263. JNCC. North-east Faroe-Shetland Channel MPA: Supplementary Advice on the Conservation Objectives (SACO). (2018).
264. Howell, K., Davies, J. & Narayanaswamy, B. Identifying deep sea megafaunal epibenthic assemblages for use in habitat mapping and marine protected area network design. Journal of the Marine Biological Association of the United Kingdom 90, 33–68 (2010).
265. ICES. A suggestive list of deep-water VMEs and their characteristic taxa. https://www.ices.dk/data/Documents/VME/VMEs%20and%20 their%20taxa.pdf (2020).
266. ICES. ICES/NAFO JOINT WORKING GROUP ON DEEP-WATER ECOLOGY (WGDEC). file:///C:/ Users/owner/Downloads ICESNAFOJointWorkingGrouponDeepwaterEcologyWGDEC_Republished.pdf (2020).
267. Indraningrat, A. A. G., Smidt, H. & Sipkema, D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Marine Drugs 14, (2016).
268. Cooley, S. et al. Oceans and coastal ecosystems and their services In: in Climate Change 2022: Impacts, adaptation and vulnerability. Contribution of the WGII to the 6th assessment report of the intergovernmental panel on climate change IPCC AR6 WGII (Cambridge University Press, 2022).
269. Daniel O. B. Jones, Ian R. Hudson, & Brian J. Bett. Effects of physical disturbance on the cold-water megafaunal communities of the Faroe–Shetland Channel. Mar Ecol Prog Ser 319, 43–54 (2006).
270. Tjensvoll I, Kutti T, Fosså JH, & Bannister RJ. Rapid respiratory responses of the deep-water sponge Geodia barretti exposed to suspended sediments. Aquat Biol 19, 65–73 (2013).
271. Henry, L.-A., Harries, D., Kingston, P. & Roberts, J. M. Historic scale and persistence of drill cuttings impacts on North Sea benthos. Marine Environmental Research 129, 219–228 (2017).
272. BBC News. Cambo oil field project ‘could jeopardise deep sea life’. BBC News (2021).
273. SICCAR POINT ENERGY. 2021. Cambo Oil Field, UKCS Blocks 204/4a, 204/5a, 204/9a and 204/10a Environmental Impact Assessment (EIA). https://assets.publishing.service.gov.uk/ government/uploads/system/uploads/attachment_data/file/991817/D-4261-2021_-_ES.pdf (2021).
274. Webster, L. et al. Monitoring of Polycyclic Aromatic Hydrocarbons (PAHs) in Scottish Deepwater environments. Marine Pollution Bulletin 128, 456–459 (2018).
275. Luter, H. M. et al. The Effects of Crude Oil and Dispersant on the Larval Sponge Holobiont. mSystems 4, e00743-19 (2019).
276. Kahn, A. S., Yahel, G., Chu, J. W. F., Tunnicliffe, V. & Leys, S. P. Benthic grazing and carbon sequestration by deep-water glass sponge reefs. Limnology and Oceanography 60, 78–88 (2015).
277. Guihen, D., White, M. & Lundälv, T. Temperature shocks and ecological implications at a cold-water coral reef. Marine Biodiversity Records 5, (2012).
278. OSPAR Commission. Background Document for Ocean quahog Arctica islandica. https:// qsr2010.ospar.org/media/assessments/Species/ P00407_Ocean_quahog.pdf (2010).
279. JNCC and Natural England. Review of the MCZ Features of Conservation Importance. https://data.jncc.gov.uk/data/94f961af-0bfc4787-92d7-0c3bcf0fd083/MCZ-review-foci201605-v7.0.pdf (2016).
280. Garcia, S. et al. Protecting the North Sea: Holderness. 32 (2019).
281. Department of Agriculture, Environment and Rural Affairs. Conservation Objectives and Potential Management Options: Outer Belfast Lough Marine Conservation Zone (MCZ). https://niopa. qub.ac.uk/bitstream/NIOPA/5164/1/Conservation%20Objectives%20and%20Potential%20 Management%20Options%20-%20Outer%20 Belfast%20Lough%20MCZ_0.pdf (2016).
282. Hawes, J., Noble-James, T., Lozach, S., Archer-Rand, S., & Cunha, A. North East of Farnes Deep Marine Conservation Zone (MCZ) Monitoring Report 2016.
283. Mazik, K, S. N. et al. A review of the recovery potential and influencing factors of relevance to the management of habitats and species within Marine Protected Areas around Scotland. (2015).
284. Offshore Petroleum Regulator for Environment and Decommissioning. Talbot Field Development. https://www.gov.uk/government/ publications/talbot-field-development (2022).
285. Steimle, F.W., Boehm, P.D., Zdanowicz, V.S., & Bruno, R.A. Organic and trace metal levels in ocean quahog, Arctica islandica Linne, from the Northwestern Atlantic. FISHERY BULLETIN 84, (1986).
286. Tyler-Walters, H. & Hiscock, K. Arctica islandica Icelandic cyprine. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews. Plymouth: Marine Biological Association of the United Kingdom. in (2017).
287. Ridgway, I. D. & Richardson, C. A. Arctica islandica: the longest lived non colonial animal known to science. Reviews in Fish Biology and Fisheries 21, 297–310 (2011).
288. Butler, P. G., Wanamaker, A. D., Scourse, J. D., Richardson, C. A. & Reynolds, D. J. Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeography, Palaeoclimatology, Palaeoecology 373, 141–151 (2013).
289. Estrella-Martínez, J. et al. Reconstruction of Atlantic herring ( Clupea harengus ) recruitment in the North Sea for the past 455 years based on the δ 13 C from annual shell increments of the ocean quahog ( Arctica islandica ). Fish and Fisheries 20, (2019).
290. Butler, P. G. et al. Is there a reliable taphonomic clock in the temperate North Atlantic? An example from a North Sea population of the mollusc Arctica islandica. Palaeogeography, Palaeoclimatology, Palaeoecology 560, 109975 (2020).
291. Arellano-Nava, B. et al. Destabilisation of the Subpolar North Atlantic prior to the Little Ice Age. Nature Communications 13, 5008 (2022).
292. Stott, K. et al. The potential of Arctica islandica growth records to reconstruct coastal climate in north west Scotland, UK. Quaternary Science Reviews 29, 1602–1613 (2010).
293. Liehr, G. A., Zettler, M. L., Leipe, T. & Witt, G. The ocean quahog Arctica islandica L.: a bioindicator for contaminated sediments. Marine Biology 147, 671–679 (2005).
294. OSPAR Commission. List of Threatened and/or Declining Species & Habitats. OSPAR Commission https://www.ospar.org/work-areas/ bdc/species-habitats/list-of-threatened-declining-species-habitats (2008).
295. Witbaard, R. & Bergman, M. J. N. The distribution and population structure of the bivalve Arctica islandica L. in the North Sea: what possible factors are involved? Journal of Sea Research 50, 11–25 (2003).
296. Ballesta-Artero, I., Janssen, R., Meer, J. van der & Witbaard, R. Interactive effects of temperature and food availability on the growth of Arctica islandica (Bivalvia) juveniles. Marine Environmental Research 133, 67–77 (2018).
297. Brey, T., Arntz, W. E., Pauly, D. & Rumohr, H. Arctica (Cyprina) islandica in Kiel Bay (Western Baltic): growth, production and ecological significance. Journal of Experimental Marine Biology and Ecology 136, 217–235 (1990).
298. JNCC. Faroe-Shetland Sponge Belt Nature Conservation Marine Protected Area: Data Confidence Assessment. https://data.jncc. gov.uk/data/411ea794-b135-4877-9fc8-e3e6c054eef9/FSSB-2-DataConfidenceAssessment-v5.0.pdf (2014).